Set language
Save
| General | |
|---|---|
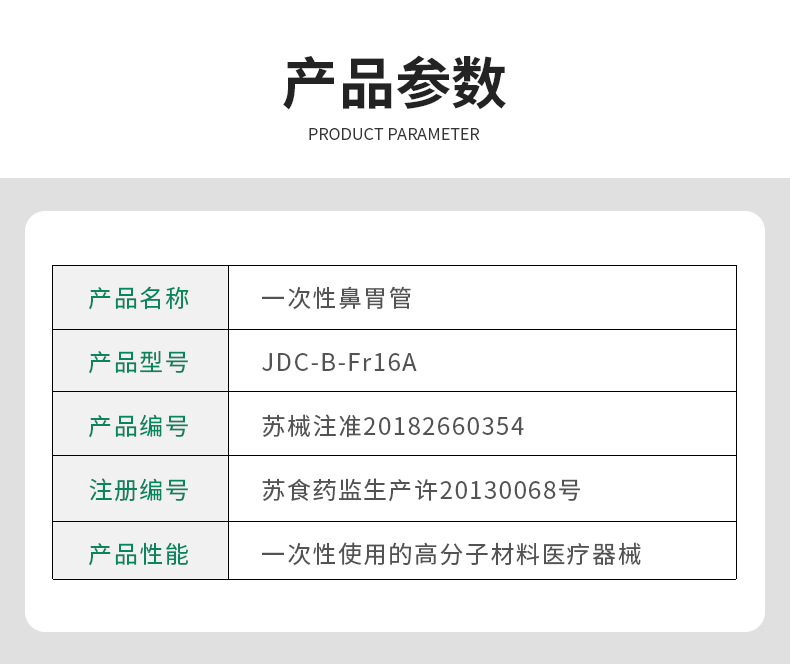
| Drug (Device) Approval Number | Medical Device Registration No. 20182140354 |
| Item number | 001 |
| Production license | Su Yao Jian Medical Device Production License No. 20130068 |
| Product Registration Certificate | Medical Device Registration No. 20182140354 |
| Sanitary Permit | ------- |
| Filing Certificate | Medical Device Registration No. 20182140354 |
| Sterilization method | High temperature |
| Validity period | 36 |
| For single use | is |
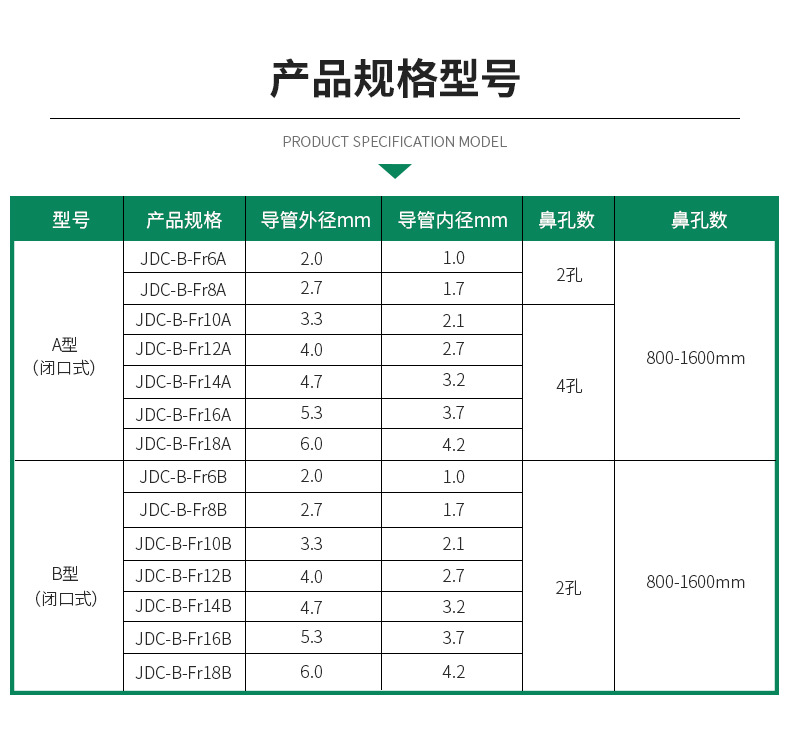
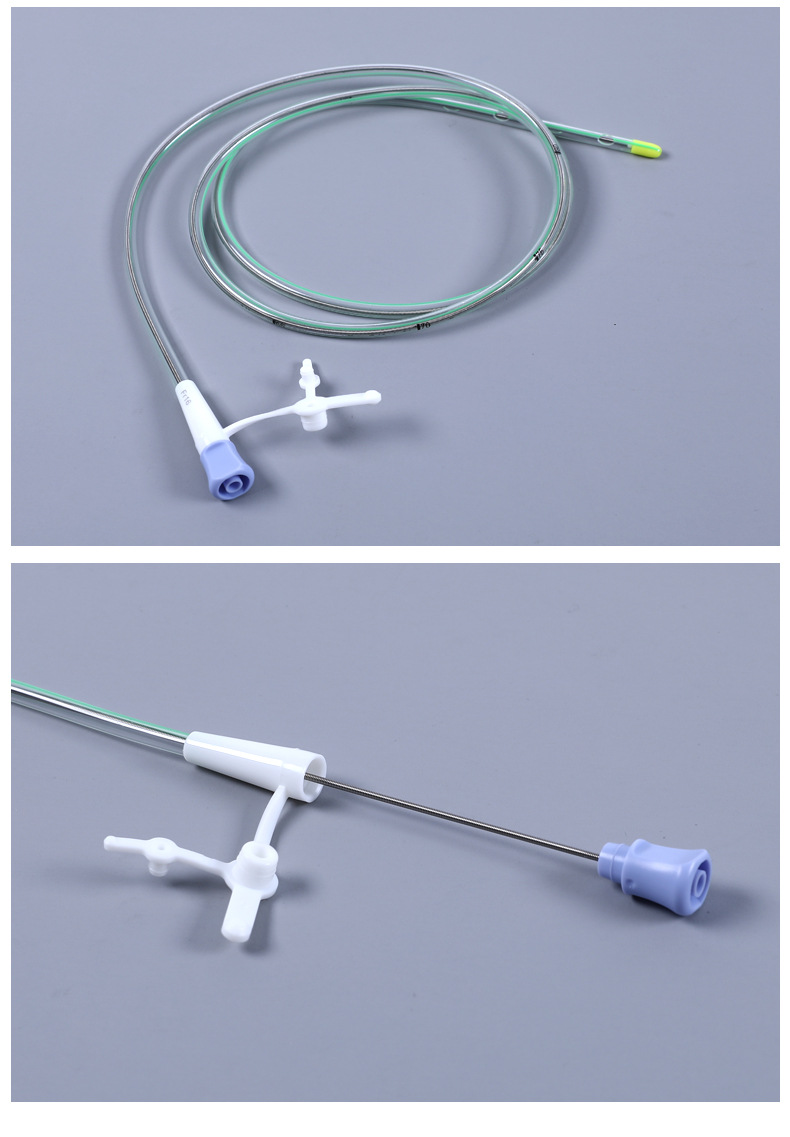
| Specification | F16 |
| Model | Double-chamber type |
| Brand | For more details, please inquire |
| Indwelling time in the body | 42 days |
| Catheter length | 1100mm in length |