Set language
Save
| General | |
|---|---|
| Drug (Device) Approval Number | Medical Device Registration No. 20172141327 |
| Item number | 1019 |
| Production license | ----- |
| Product Registration Certificate | Medical Device Registration No. 20172141327 |
| Sanitary Permit | --------- |
| Filing Certificate | Medical Device Registration No. 20172141327 |
| Sterilization method | High temperature |
| Validity period | 24 |
| For single use | is |
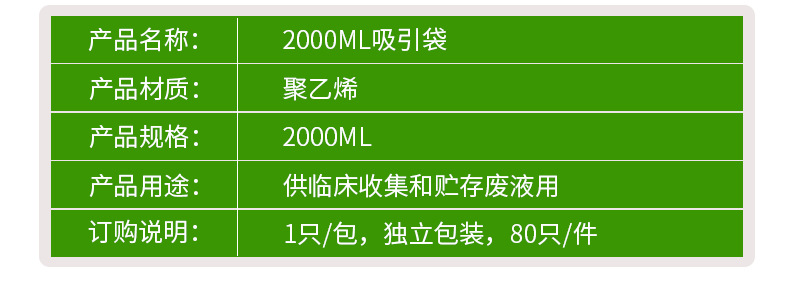
| Specification | 2000ml |
| Features | There is a specimen collection port |
| Brand | For more details, please inquire |