Set language
Save
| General | |
|---|---|
| Drug (Device) Approval Number | Medical Device Registration No. 20162141217 |
| Item number | 1040 |
| Production license | Su Food and Drug Administration Medical Device Production License No. 20120073 |
| Product Registration Certificate | Medical Device Registration No. 20162141217 |
| Sanitary Permit | ------- |
| Filing Certificate | Medical Device Registration No. 20162141217 |
| Sterilization method | Ethylene oxide |
| Validity period | 24 |
| For single use | is |
| Specification | Double-headed |
| Model | F5 F6 F7 F8 |
| Brand | Culture and education |
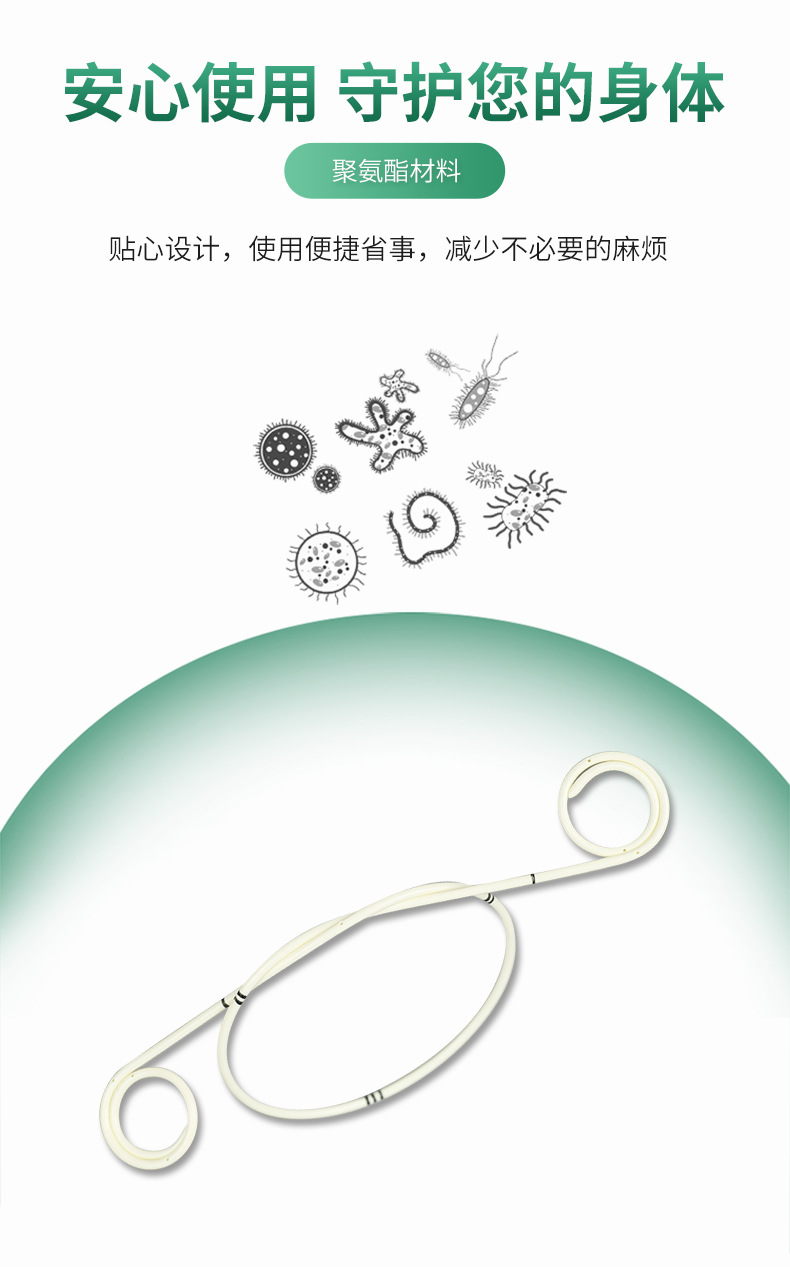
| Material | TPU polyurethane material |