Set language
Save
| General | |
|---|---|
| Drug (Device) Approval Number | Medical Device Registration No. 20162081478 |
| Item number | 1032 |
| Production license | Su Food and Drug Administration Medical Device Production License No. 20120073 |
| Product Registration Certificate | Medical Device Registration No. 20162081478 |
| Sanitary Permit | ---------- |
| Filing Certificate | Medical Device Registration No. 20162081478 |
| Sterilization method | Ethylene oxide |
| Validity period | 24 months |
| For single use | is |
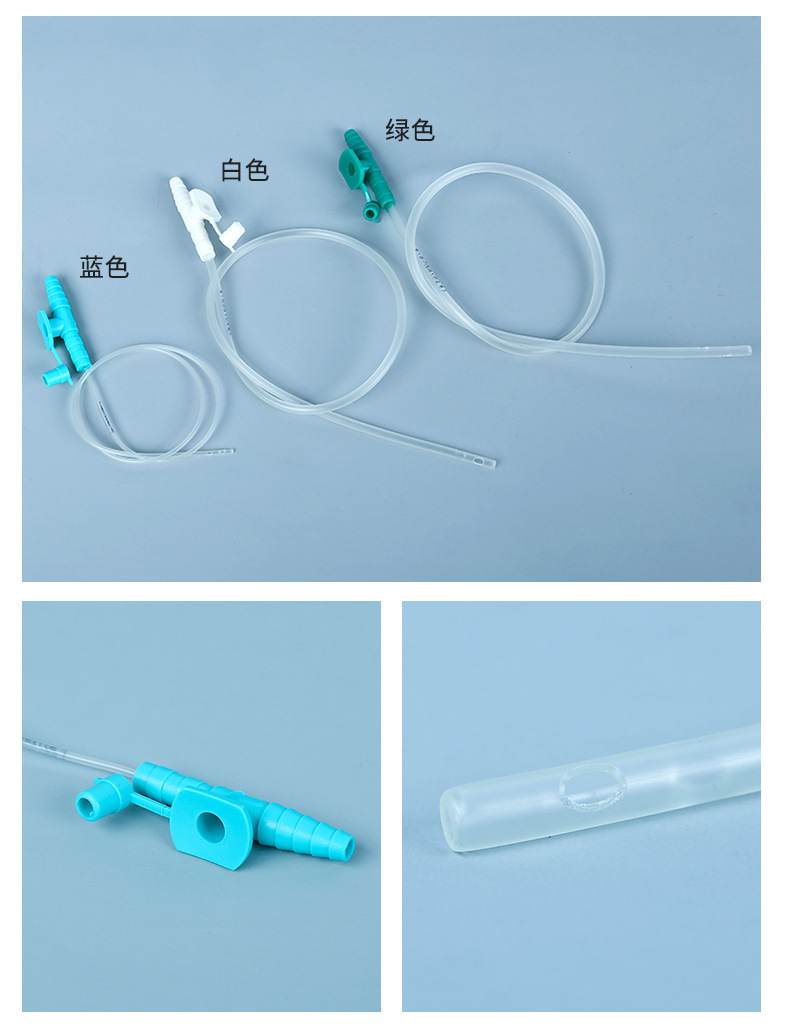
| Specification | F6,F8,F10,F12,F14 |
| Model | Arc-shaped joint |
| Brand | Culture and education |
| Purpose | For clinical sputum aspiration |
| Function | Controllable type |